
Balance = Stability
One of the things noted in early studies of the nucleus was the dominance of EVEN numbers of nucleons in stable nuclei. There are far more stable nuclei with EVEN numbers of nucleons than with odd numbers of nucleons. For example:
- There are 159 stable nuclei with an EVEN number of protons and an EVEN number of neutrons.
- There are 53 stable nuclei with an EVEN number of protons and an odd number of neutrons.
- There are 50 stable nuclei with an odd number of protons and an EVEN number of neutrons.
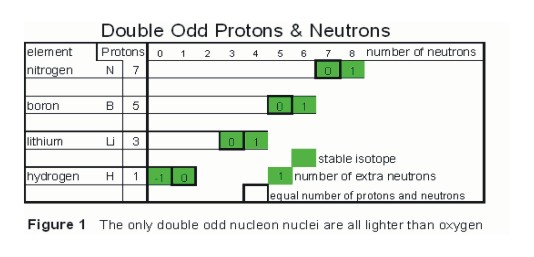
- There are 4 stable nuclei with an odd number of protons and an odd number of neutrons, and these are in nuclei lighter than oxygen and all exist in that part of the “Path of Stability” that is built using the “Deuteron Step.”
Definitions
“Valley of Stability” involves or contains all the stable nuclei of all the elements.
“Path of Stability” involves the connected stable nuclei in the Valley of Stability.
“Satellite isotopes” or nuclei are stable nuclei not connected to the Path of Stability and reside unattached and adjacent to the Path of Stability within the Valley of Stability.
Along the Path of Stability there are gaps or open steps that exist in the continuous flow of stable nuclei. These gaps always involve odd numbers of nucleons.
- There are "two proton gaps" in the Path of Stability both of which involve an odd number of protons. The first proton gap occurs at technetium with 43 protons and the second proton gap occurs at promethium with 61 protons.
- There are "ten neutron gaps" along the Path of Stability which also occurs at location with odd numbers of neutrons. These neutron gaps occur at 19, 21, 35, 39, 45, 61, 71, 89, 115 and 123 neutrons.
The two proton gaps are highlighted in RED
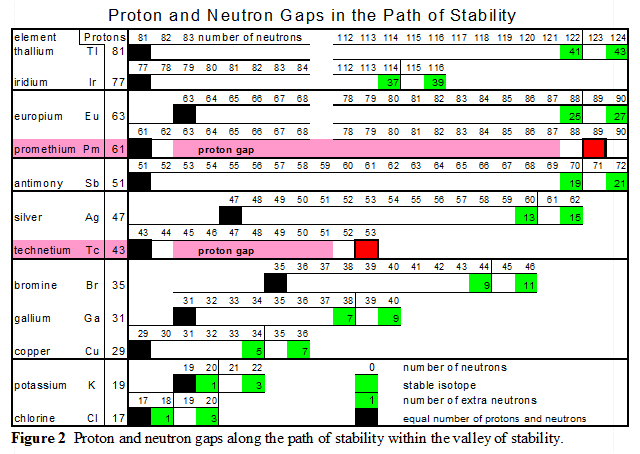
All the skipped numbers are odd. This is true for both protons and neutrons.

Neutron & Proton Gaps
- Chlorine at 19 neutrons
End of The Core Start of The Star - potassium at 21 neutrons
- copper at 35 neutrons
- Gallium at 39 neutrons
End of The Star Start of The Core Extension I - Bromine at 45 neutrons
- Then at technetium there is a gap at 43 protons

Start of The Loops I - Silver at 61 meutrons
- Antimony at 71 neutrons
- Then at promethium there is a gap at 61 protons
Start of The Loops II - Europium at 89 neutrons
- Iridium at 115 neutrons
- Thallium at 123 neutrons
The importance of even numbers of nucleons in a nucleus suggest some type of radial symmetry. It is easier to achieve balance using even numbers of particles than using odd numbers of particles. When building the nuclear lattice structure even numbers of nucleons are an essential requirement for stable nuclei.
Balance and Symmetry
This indicates that the number 2 as a very important “magic number” for nuclei. The next two important magic numbers for the nucleus are 4 and 6.
- 2 even numbers represent balance and symmetry.
- 4 is the number of nucleons to make an alpha particle 2 protons and 2 neutrons. This is the most important structure in the nucleus.
- 6 The hexagonal structure of the lattice results in partial closure every 6 protons or multiples their of. The first important closure is carbon 12. This completes the carbon ring which is the second most important lattice structure in the nucleus.
Balance = Stability in Nuclear Growth Patterns
There are many clues about balance and symmetry that can be deduced from nuclei that are within the Valley of Stability. Following are three stable nuclear growth patterns that provide clues about how and why stable nuclei occur.
|
The Deuteron Step The first pattern is the deuteron step. For more information see the section on the “Deuteron Step” for more information. This pattern only occurs during the building of the first 7 elements. Alternating protons and neutrons indicates the need for balance and the necessity of symmetry right from the start. The deuteron step add nucleons one at a time which results in an even number of isotopes for the elements in this part of the path of stability. |
 |
|
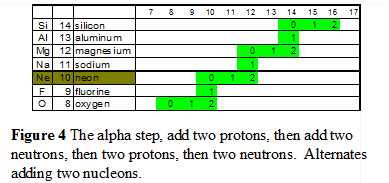
The Alpha Step The second pattern is the alpha step. See the section on the “Alpha Step” for more information. This pattern occurs during the building of the elements between oxygen and argon. It is defined by alternating between adding two protons and then adding two neutrons. The alpha and alpha+2 steps add nucleons in even quantities, thus there are an odd number of isotopes for those elements in even number of protons. The nucleus uses this schema to maintain radial balance. |
 |
|
The Alpha+2 Step The third pattern is the alpha+2 step. Also see the section on the “Alpha Step” for more information about the alpha step+2. There are always even numbers of like nucleons added before the type of nucleon is changed. This even number requirement is a result of the need to maintain balance. Where there is a neutron gap, and for those elements that have an odd numbered of proton and which cross that gap, those elements always have 2 isotopes. |
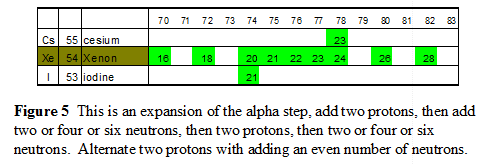 |
Balance = Stability in Stable “Satellite Isotopes.”
|
Xenon is a good example, xenon has four stable “satellite” isotopes labled with (16, 18, 26 & 28) more neutrons than protons. There are five stable isotopes on the Path of Stability (20, 21, 22, 23 & 24) extra neutrons, isotope 20 represents the second of the 2 protons added in the 1,2 proton additions of an alpha step along the path of stability. The numbers 21, 22, 23 and 24 are 4 neutrons added for an alpha+2 step along the path of stability. The total is thus nine stable isotopes of xenon in the Valley of Stability. |
 |
- Stable satellite isotopes only occur in elements with an even number of protons.
- Stable satellite isotopes always contain an even number of neutrons.
Isotopes adjacent to the Path of Stability are the result of adding or removing neutrons. When only one neutron is added or removed the nucleus that is left is unbalanced, which results in instability. However, add or remove two neutrons from a nucleus and in some cases sufficient balance is maintained that a stabile isotope of the element results. These are what are being labled “satellite” isotopes.
There is always a gap of one neutron between nuclei of a satellite nucleus and nuclei on the path of stability and between two stable satellite nuclei. There are no adjacent satellite isotopes.
This indicates that neutrons are added symmetrically around the center of the nuclear structure. Thus to maintain balance when two neutron or two protons are added to a nucleus, the two nucleons always form a straight line that passes through the center point of the nuclear structure.
Balance = Stability, Results in Dominance of Even Numbers of Nucleons
Even proton number nuclei always have an odd number of connected stable isotopes why?
- The first isotope for a nucleus, on the path of stability, that have an even number of protons is the result of the proton that was added to the nucleus of the previous elements that had an odd number of protons.
- Because the first isotope is created by adding a proton to make the next element the first isotope, it is not the result of an added neutron. Thus, subsequently an even number of neutrons are added to maintain balance in the nuclear lattice. Thus, one plus on even number of neutrons results in an odd number of isotopes . So, when two neutrons are added it results in three connected stable isotopes, adding four neutrons results in five connected stable isotopes and adding six neutrons results in seven connected isotopes or the element �tin� which has the most connected isotopes along the Path of Stability. These neutrons are required to balance the electrical forces between quarks in the nucleons before any more protons can be added to a growing nucleus.

This necessity to build the nucleus in even number of nucleons indicates that the growth must be symmetrical. This is evident because nuclei with odd numbers of protons have minimal numbers of isotopes. Varity in stable isotopes always occurs in nuclei with an even number of protons and outliers like satellite isotopes always involve even number of both protons and neutrons.
Balance = Stability, Two Even Numbered Neutron Isotopes Surround Neutron Gaps
Elements that have nuclei with an odd number of protons generally have one isotope except in two special cases where they have two isotopes.
- The first is among the first seven elements where the nucleus is built using the deuteron step.
- The second are at neutron gaps for elements heavier than oxygen.
First
Odd number of proton nuclei created by the deuteron step. That involves all odd number of proton nuclei that are lighter than oxygen, see figure 1.
Second
Odd number of proton nuclei that straddle a neutron gap have two stable isotopes, see figure 2.
When a nucleus with an odd number of protons has two stable isotopes, the stable nuclei are always separated by an unstable isotope with an odd number of neutrons. Thus, both stable isotopes have an even number of neutrons. For elements heavier than oxygen there is no stable isotope with an odd number of protons and an odd number of neutrons.
The first two sets of neutron gaps, 19 & 21 and 35 & 39, provide valuable incite into balance within the nucleus
Completion of The Core Phase
- The first stage of building a stable nucleus is the alpha particle
- The second stage in building the first carbon ring.
- Next milestone is two carbon rings which form magnesium.
- Three carbon rings which complete the initial building of the core with argon.
Completion of the initial core is where the first set of neutron gaps occurs. These two gaps occurs at 19 and 21 neutrons.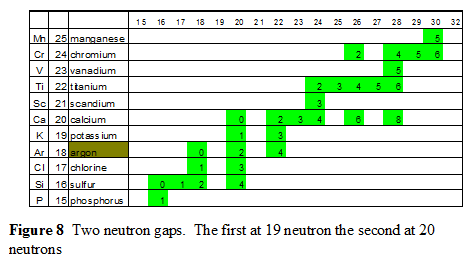
- The next stage is building the star phase that culminates with zinc.
Completion of The Star Phase
Completion of the star phase is where the second set of neutron gaps occurs. These two gaps occur at 35 and 39 neutrons.
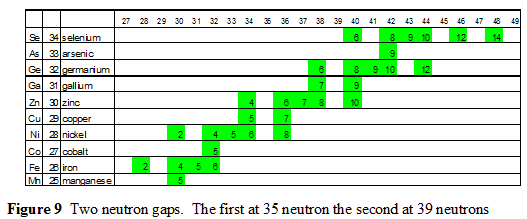
These two sets of gaps occur at the transition between phases of building the nucleus. The first set occurs at the transition between the initial building of the Core Phase and the starting of the Star Phase. The second set occurs between the Star Phase and the commencement of the Loops Phase. The next neutron gaps 5 thru 8 at 45, 61, 71 & 89 neutrons and the two proton gaps at 43 & 61 protons are related to transitions in the Loops Phase. All these gaps provide clues about a slight skew in the shape of the six sided of the alpha particle. This skew is further indicated by the nucleon binding energy which peaks at nickel, two elements before the lattice closes at zinc.
This skewness results from differences in the magnitude of the electric poles and magnetic dipoles of the quarks which creates a slight warping of the symmetry of the six sided alpha particle. Thus when a phase closes or the structure of the nuclear lattice closes slight adjustment is needed which reduce the energy that would be available if the final nucleons slid in without the need for these accommodations in the lattice structure.
All the above examples relate to the balance and symmetry that exist within stable nuclei. This symmetry is with respect to the center point of the nucleus. Balance indicates that nucleons are added in a fashion that will minimize “vibration” within the nucleus.