
DEUTERON STEP
Three nuclear growth patterns are discussed that provided the first clues that made it possible to decipher nuclear structure.
The three patterns are
- The Deuteron step
- The Alpha Step (lighter elements)
- The Alpha Step + 2 (heavier elements)
These patterns resemble the addition of precise parts to a regular structure and adding these parts in a specific order so that a very well defined structure is assembled. These growth patterns provided the first clues that made it possible to decipher nuclear structure.
How growth of stable nuclei occurs becomes obvious when these three nuclear growth patterns are examined plus why growth occurs along the Valley of Stability from one element or isotope to the next. This suggest that rather than just a few structure closures at the magic numbers there are many closures along the path of stability.
Understanding this path and how it grows is significant and is the key to understanding nuclear structure.
- These growth patterns resemble building a very specific structure and maintaining a delicate balance while building that structure.
- The positions of the proton and neutron gaps along the path of stability indicate points where bad balance or instability exists. At these points of instability, two protons or two neutrons need to be added at the same time to maintain stability or balance.
- The final clues came by answering the question, �Why can't an unlimited number of electrically neutral neutrons be added to or placed in the nucleus?�
THE DEUTERON STEP is the first primary method used to build stable nuclei for elements lighter than oxygen. The deuteron step adds neutrons and protons alternatively to form the next stable nucleus.
The only part of the path of stability where the deuteron step occurs is between hydrogen and oxygen, see graph below.
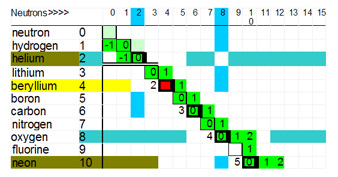
Example: the Deuteron Step as it exists between boron and oxygen is as follows:
- Boron10 has 5 protons and 5 neutrons, what single nucleon can be added to boron10 to form a stable nucleus? A proton - NO. A neutron - YES. This makes boron11.
- Boron11 has 5 protons and 6 neutrons, what single nucleon can be added to boron11 to form a stable nucleus? A proton - YES. A neutron - NO. This makes carbon12.
- Carbon12 has 6 protons and 6 neutrons, what single nucleon form a stable nucleus? A proton - NO. A neutron - YES. This makes carbon13.
- Carbon13 has 6 protons and 7 neutrons, add a proton to create nitrogen14.
- Nitrogen14, add a neutron to make nitrogen 15, and then add a proton to make oxygen16.
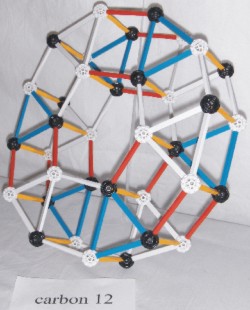
Carbon consists of three alpha particles and completes the first ring of the nuclear core.
There is a deuteron step switch between helium4 and lithium6, thus there is no element or isotope with and �A� of 5. For "A" of 8 is another unsupportable isotope number.
The nuclear structure from hydrogen to boron is very interesting especially for beryllium.
These processes are explained in detail in the section, hydrogen1 to carbon12.