
Spin & Magnetic Dipole
The nuclei of atoms have six important physical properties that dictate their attributes. The first is mass, second is the exclusion principle, the third is the strong force fourth is the electric field, fifth magnetic dipole, and the sixth is spin.
Atomic mass resides primarily in the nucleus. Mass generally determines the general bulk properties of the element such as weight, density, size and other related material attributes.
The exclusion principle keeps the nucleus from collapsing into a very small black hole.
The strong nuclear force principally holds the nucleus and nucleons together, thus a sort of very strong nuclear �gravity.�
The nuclear electric charge determines element creates a positive potential well around which the electron shells can develop, the number of electrons and their distribution around the nucleus. The nucleus is responsible for the number of electron shells and number of electrons in each of those shells is determined by the nucleus. The macro charge of the nucleus is in reality responsible for the chemical properties of the various elements by determining the ultimate configuration of the electrons involved in chemical reactions. The micro electrical charge that exists at the nuclear scale is partially responsible for nuclear lattice structure.
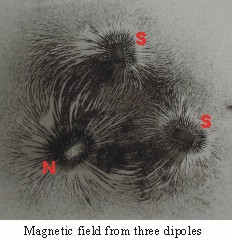
The nuclear magnetic dipole interacts with external magnetic fields. The magnetic dipole of nuclei is always the same for any given element. This consistency speaks volumes about the consistency within the nucleus. The macro magnetic field of combined local magnetic dipoles in the nucleus is responsible for the magnetic dipole of the nucleus. The micro magnetic dipoles at the nuclear scale are also partially responsible for nuclear structure.
Nuclear Spin is probably the most perplexing or nebulous property of the nucleus, yet it is a property that is absolutely consistent, does not vary and is always the same value for all the nuclei of a given element or isotope. Again the consistency of spin speaks volumes about the stability of all components within the nucleus.
The micro field of the electrical charge that occurs at the micro nuclear scale in conjunction with nuclear micro magnetic field resulting from the magnetic dipoles and particles spin are ultimately responsible for nuclear lattice structure.
Nuclear Mass, the Strong Force, the Exclusion Principle
Nuclear mass is a function of the number of protons and neutron and the binding energy holding them within the nucleus. That binding energy resides in a balance between the strong mesonic force, the strong color force, the magnetic dipoles, the electric charges, the exclusion principle and spin. These pressures within the nucleus provide both stability and instability because they are in constant conflict to provide balance within the nucleus between size, cohesiveness and ultimately nuclear structure.
Nuclear Spin
Nuclear spin at instances is part of the exclusion principle. Spin also is tied to the magnetic dipole and the two properties are aligned. The equation defining this relation ship is:
where
μ and μ are the magnetic dipole
moment, and
μ is a vector.
J and j are the spin quantum number,
and
J is a vector.
h is planks constant.
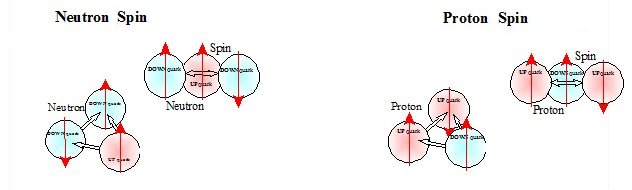
Deuteron Spin
Spin of the deuteron is a complex problem. Generally when a nucleus has an even number of nucleons the spin is 0 at the ground state. Most stable nuclei have a spin of "0" or "n/2". The four odd, odd light nuclei are the exceptions. Deuterium2 has a spin=1, even though A=2, because there is only "1" neutron and "1" proton.
Deuterium's spin is 1 because there are not an equal number of up and down quarks pairs. In the deuteron there are 1.5 pairs of down quarks and 1.5 pairs of up quarks. In the alpha particle or helium nucleus there are 3 pairs of up quarks and 3 pairs of down quarks. Individual quarks have a spin=1/2 but for pairs of like quarks the spin is generally zero. When there is an even number of like quarks in the nucleus they can form pair which form coupled magnetic dipoles that align to cancel each others magnetic fields and spin. This is because the magnetic field is inextricably connected to spin. Thus, this alignment of the two magnetic dipoles and spins will cancel resulting in 0 spin and a 0 magnetic dipole. Otherwise without an even number of quark pairs there are residual magnetic dipoles and residual spins that are not canceled.
Along the path of stability all the odd, odd nuclei are all created during the deuteron step building phase and are thus all lighter than oxygen. The four odd, odd nuclei are, deuterium2 spin=1, lithium6 spin=1, boron10 spin=3, and nitrogen10 spin=1. Generally if both the number of protons and the number of neutrons in a nucleus are an odd number then the spin ground state is not 0 but is an integer.
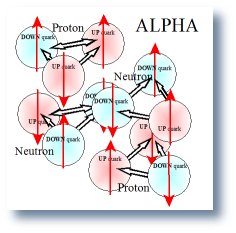
The ALPHA Spin � Zero
Spin of the alpha particle is 0 in the ground state.
Nuclear Magnetic Dipoles
The model shown below was made using three bar magnetic to represent the three magnetic dipole of the three quarks in a proton or neutron. Iron filings were used to outline the shape of the resulting magnetic field. This model shows how the two south poles repel, while individually coupling both south poles with the single north pole. Also shown are attachments that outline the magnetic fields parallel to the three dipole pairs. Two pairs couple and the third pair repels.
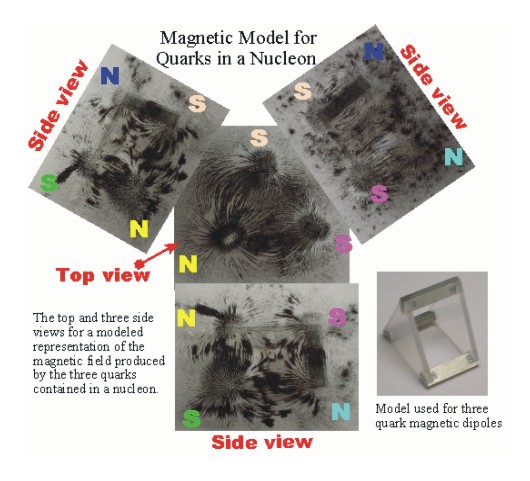
In the bottom right hand corner is a picture of the three bar magnets and the arrangement used for this model.
Alignments of the magnetic dipoles in the nucleus are the results of interaction of the magnetic fields of the individual quarks. The ultimate field is defined by the principle of superposition which provides a method for calculating of complex magnetic fields through simple vector addition of the individual magnetic fields produced by the individual quark dipoles that are involved in generating the complete and complex magnetic field.
Superposition is based on the consevation of energy. Thus, superposition is valid for magnetic field, for gravity and for electric fields, and because it is based on the conservation of energy, superposition even works for quantum mechanics. Thus, the principle of superposition provides a rapid, amazingly beautiful and simple way to analyze the magnetic field or any other field generated by the quarks in the nucleus.
- At atomic or macro distance for the nucleons such as at electron shell distance, the magnetic field can be described as a single or isolated dipoles within the nucleus.
- At local, micro or nuclear distance, that is within the nucleus itself, the magnetic fields interacts and express both effective and complex aspects of the multiple dipole fields present and the interactions with the other magnetic dipoles that are in the nucleons.
- In most cases the magnetic field of the nucleus is not a simple multiple of the quark dipoles. This is because the magnetic field is a vector field because the magnetic field adds vectorially. This imbalance is very important in understanding how mechanical balance is maintained in nuclei that have an odd number of nucleons.
4. Analyzing the far magnetic field of the proton the field generated by the two up quarks cancel leaving only the magnetic field generated by the dipole of the down quark. Analyzing the far magnetic field of the neutron the field generated by the two down quarks cancel leaving the magnetic field generated by the up quark�s magnetic dipole.
Thus, using the law of superposition it can be concluded that:
- the magnetic dipole of the proton equals the magnetic dipole of the down quark.
- the magnetic dipole of the neutron equals the magnetic dipole of the up quark.
| 1. Magnetic field of the Proton | 2. Magnetic field of the Neutron | 3. Magnetic field of the Deuteron |
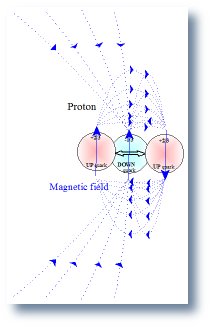
|
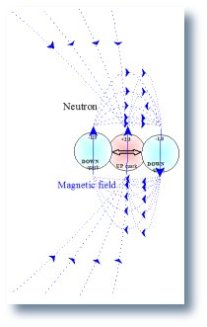
|
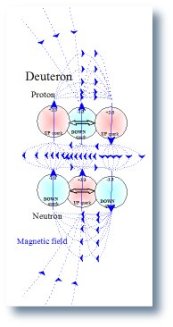
|
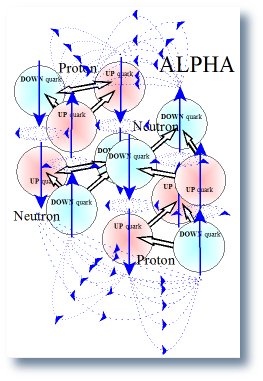
Magnetic field of the ALPHA particle
The included illustration of the alpha particle shows all the magnetic dipoles and some of the magnetic field line generated by those dipoles. All the field lines are not included because the picture would become so cluttered that it would be un-readable. The reader can easily continue the scheme and demonstrate that the macro magnetic field of the alpha will be zero but in local or field within and very close to the nucleus will be complex. Thus, it is left to the readers, if they desire, to fill in all the magnetic field line for the alpha particle. The result is that all interconnections cancel the far or macro magnetic field just as predicted by the law of superposition.
The interactions of all these dipoles and their accompanying fields help create the ultimate structure in the nucleus.
Nuclear Electric Charge
The effective electrical charge of the nucleus at the distance of the electron shells is propositional to the number of protons in the nucleus. The nuclear electric charges or number of protons in the nucleus determines the element. The nucleus is the central force that the electron shell structure of the atom is built around. Electrical charge of the nucleus determines the chemical properties of the element by regulating three properties:
- first the number of electrons normally associated with the atom,
- second the number of electron shells and
- third the number of electrons that can reside in those shells.
Thus the charge of the nucleus determines whether an atom can accept additional electrons to fill a partially filled shell or will give up electrons from a partially filled shell. This points out one of the interesting problems with the nuclear shell model because there is no central force in the nucleus around which to build any type of nuclear shell structure.
As stated in the magnetic dipole section above the principle of superposition provides for the calculation of complex fields through simple vector addition. This principle can also be used for the nuclear electric fields. Thus, the principle of superposition provides a rapid and simple way to analyze the electric field generated by the quarks in the nucleons and nucleus.
- At atomic or macro distance for the nucleons such as at electron shell distance, the electrical field is simply equal to the electrical sums and differences of the electric charges of the up and down quarks in the nucleus, which yields the same results as simply adding up the number of protons in the nucleus.
- At local or micro distance, within the nucleus itself, the electric field is controled by the electric fields of the quarks.
- The magnetic fields interact and express both effective and complex aspects of the multiple dipole fields present and the interactions with the other magnetic dipoles that are in the nucleons.
On the local level or at nuclear distances the nucleons display charge. The proton is built from three charged particles two up quarks with a charge of +2/3 and one down quark with a charge of -1/3 that yields a plus one charge at a distance proportional to electrons. The neutron is also built from three charged particles one up quark with a charge of +2/3 and two down quarks with a charge of -1/3 that yields a zero charge at a distance proportional to electrons.
Just as with spin and magnetic dipole the local electric field is stable and does not change having the ability to impose structure just as the electric field imposes structure in crystals and other lattice atomic and molecular structures. This structure will become ever more evident in the following sections.