
Radius & Binding Energy
Binding Energy
The binding energies of the elements from hydrogen A=1 to silicon A=28 show peaks when the nucleon count equals a whole number of alphas. This is even true for Beryllium A=8 even though its total binding energy is less than two alphas. Because the binding energy of beryllium8 is lower than two alphas� it degenerates to the more stable state of two alpha particles. This binding energy suggested that alpha particle might be the basic building blocks of the nucleus. Thus one of the early nuclear models was based on the alpha particle.
The alpha cluster model was proposed because of the peaks on the binding energy graph of the lighter element and the fact that the dominant decay mode heavier elements is via alpha particles. Additionally several high energy bombardment experiments found that for medium and large nuclei an inexpediently large number of alpha and multiple alpha particle fragments were found in the ruminants left after high energy collisions.
The shortfall of the cluster model was the extra neutrons in the nucleus for elements heavier than argon A=36. In later stages of nuclear growth protons that would terminate a lattice column are capped by a neutrons. These caps shield the ends of the columns and explain all the extra neutrons that are found in the nucleus.
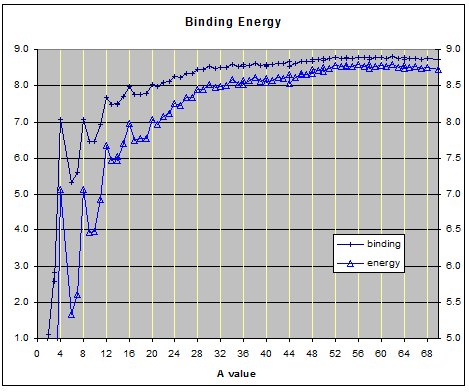
The above graph shows the binding energies of the elements from hydrogen A=1 to germanium a=70.
While observing the growth of the nuclear lattice structure through the core phase of the nucleus it becomes evident the binding energy can be used to predict the next position that the next nucleon will most likely occupy in the lattice structure. This is especially evident for elements heavier than carbon.
Nuclear Radius - Measured verses Model
The average radius of a select number of radius are compared in the following graph.
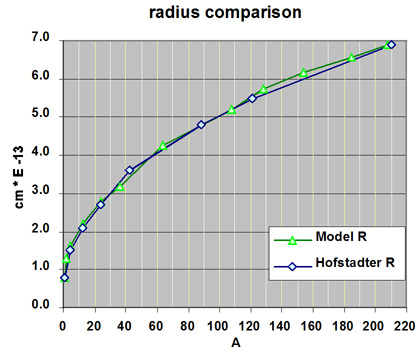
The average measured radius of this model are compared with the nuclear radius determined by R. Hofstadter using charge density. The model radii are averaged from measurements made on a model constructed from ZOME parts. The radius of the model was obtained by measuring the length along the three axis and then calculating the average radius. Size measurements made for the model were normalized to the size of the proton or the hydrogen nucleus.